5dz2 electron orbital, illustration
Bildnummer 14245022
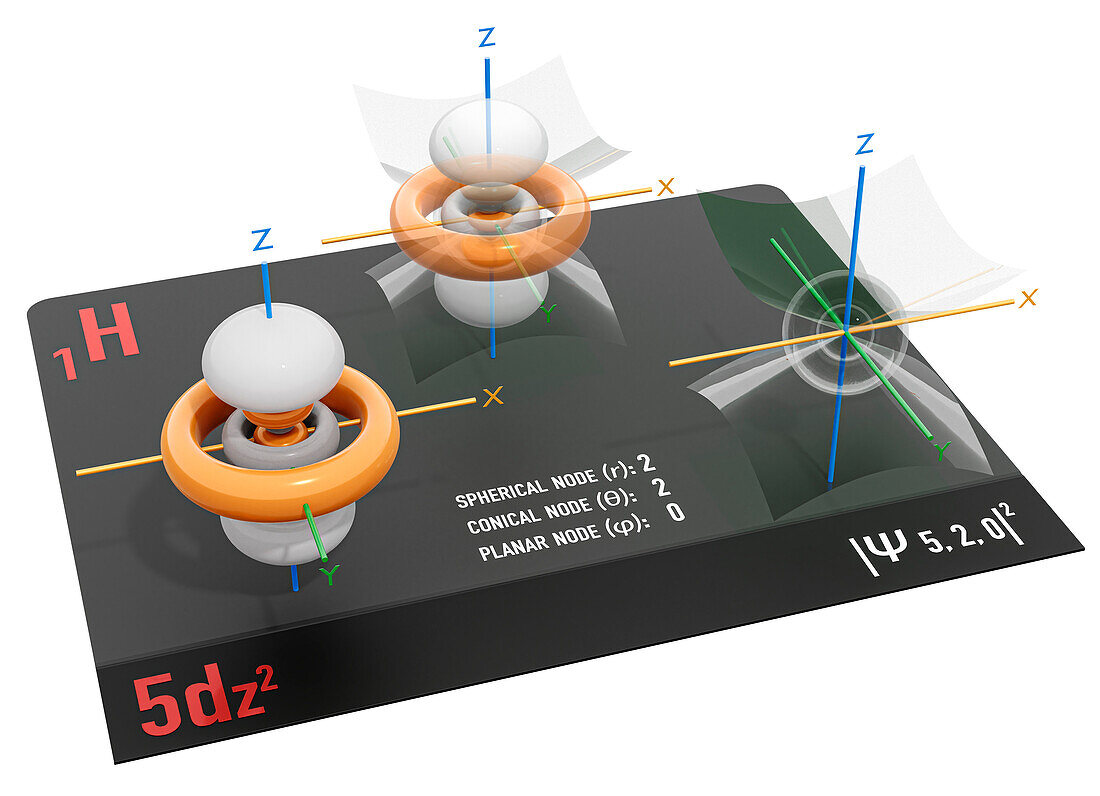
| 5dz2 electron orbital, illustration. An electron orbital is a region around an atomic nucleus (not seen) in which one or a pair of electrons is most likely to exist. The 5dz2 orbital has a unique shape consisting of nine lobes elongated along the z-axis with a doughnut-shaped region (or torus) around the nucleus on the x-y plane. The orbital is seen transparent at middle to show the axes of symmetry and the conical nodes and spherical nodes can be seen at right. Nodes are the regions in an atom with zero electron density and where the electron is least likely to exist. For the 5dz2 electron orbital, '5' indicates that it is the fifth energy level, 'd' indicates that the orbital is specifically a d-orbital, and '(z2)' indicates that the lobes of the orbital are oriented along the z-axis. The 5dz2 orbital can accommodate up to 2 electrons. It is part of the 5d shell, which contains five orbitals in total. The 5d orbitals are part of the 5 shell, which also contains one spherical 5s orbital and three bi-lobed 5p orbitals, both at a lower energy, and seven lobed 5f orbitals at a higher energy level (not seen). | |
| Lizenzart: | Lizenzpflichtig |
| Credit: | Science Photo Library / Clarivan, Carlos |
| Bildgröße: | 4920 px × 3559 px |
| Modell-Rechte: | nicht erforderlich |
| Eigentums-Rechte: | nicht erforderlich |
| Restrictions: | - |
Preise für dieses Bild ab 15 €
Universitäten & Organisationen
(Informationsmaterial Digital, Informationsmaterial Print, Lehrmaterial Digital etc.)
ab 15 €
Redaktionell
(Bücher, Bücher: Sach- und Fachliteratur, Digitale Medien (redaktionell) etc.)
ab 30 €
Werbung
(Anzeigen, Aussenwerbung, Digitale Medien, Fernsehwerbung, Karten, Werbemittel, Zeitschriften etc.)
ab 55 €
Handelsprodukte
(bedruckte Textilie, Kalender, Postkarte, Grußkarte, Verpackung etc.)
ab 75 €
Pauschalpreise
Rechtepakete für die unbeschränkte Bildnutzung in Print oder Online
ab 495 €
