Graphite molecular structure, illustration
Bildnummer 12584407
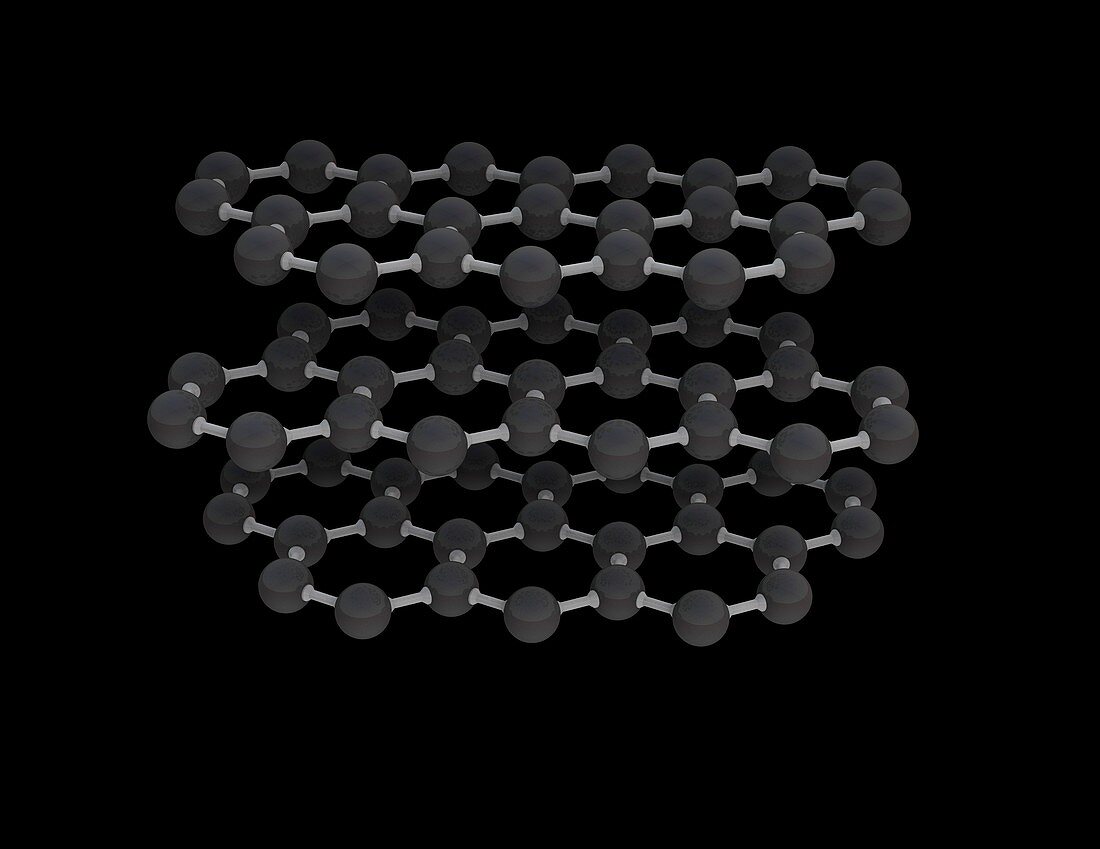
| Graphite molecular structure, illustration. Graphite is a form (allotrope) of the element carbon. Graphite is used in pencil leads and as a lubricant. It is composed of parallel layers of hexagonally arranged carbon atoms (spheres). Within each layer the carbon atoms are linked by strong covalent bonds, while the parallel layers are linked together by weak Van der Waals' forces. This Van der Waals' bonding is strong enough to hold the layers together, yet weak enough to let them slide over each other. This results in graphite's softness and its ability to act as a lubricant. For illustrations showing the hexagonal unit cell, see images C042/4538 to C042/4541. | |
| Lizenzart: | Lizenzpflichtig |
| Credit: | Science Photo Library / Jensen, Mikkel Juul |
| Bildgröße: | 6698 px × 5171 px |
| Modell-Rechte: | nicht erforderlich |
| Eigentums-Rechte: | nicht erforderlich |
| Restrictions: | - |
Preise für dieses Bild ab 15 €
Universitäten & Organisationen
(Informationsmaterial Digital, Informationsmaterial Print, Lehrmaterial Digital etc.)
ab 15 €
Redaktionell
(Bücher, Bücher: Sach- und Fachliteratur, Digitale Medien (redaktionell) etc.)
ab 30 €
Werbung
(Anzeigen, Aussenwerbung, Digitale Medien, Fernsehwerbung, Karten, Werbemittel, Zeitschriften etc.)
ab 55 €
Handelsprodukte
(bedruckte Textilie, Kalender, Postkarte, Grußkarte, Verpackung etc.)
ab 75 €
Pauschalpreise
Rechtepakete für die unbeschränkte Bildnutzung in Print oder Online
ab 495 €
Keywords
- atomare Struktur,
- Atome,
- ausgeschnitten,
- Ausschnitte,
- Bindung,
- Chemie,
- chemisch,
- Einfarbig,
- Element,
- Erscheinungsform,
- Geschichtet,
- Grafik,
- Illustration,
- Kohlenstoff,
- Kunstwerk,
- Makromolekül,
- Materialwissenschaft,
- Mineralogie,
- Modell-,
- Molekül,
- Molekular,
- molekulare Struktur,
- Niemand,
- Organisch,
- Sanft,
- Schichten,
- Schwarz und weiß,
- schwarzer Hintergrund,
- Sechseckig,
- Struktur,
- Verbindung,
- Verbindungen
