Electrical conductivity,1 of 3
Bildnummer 12016997
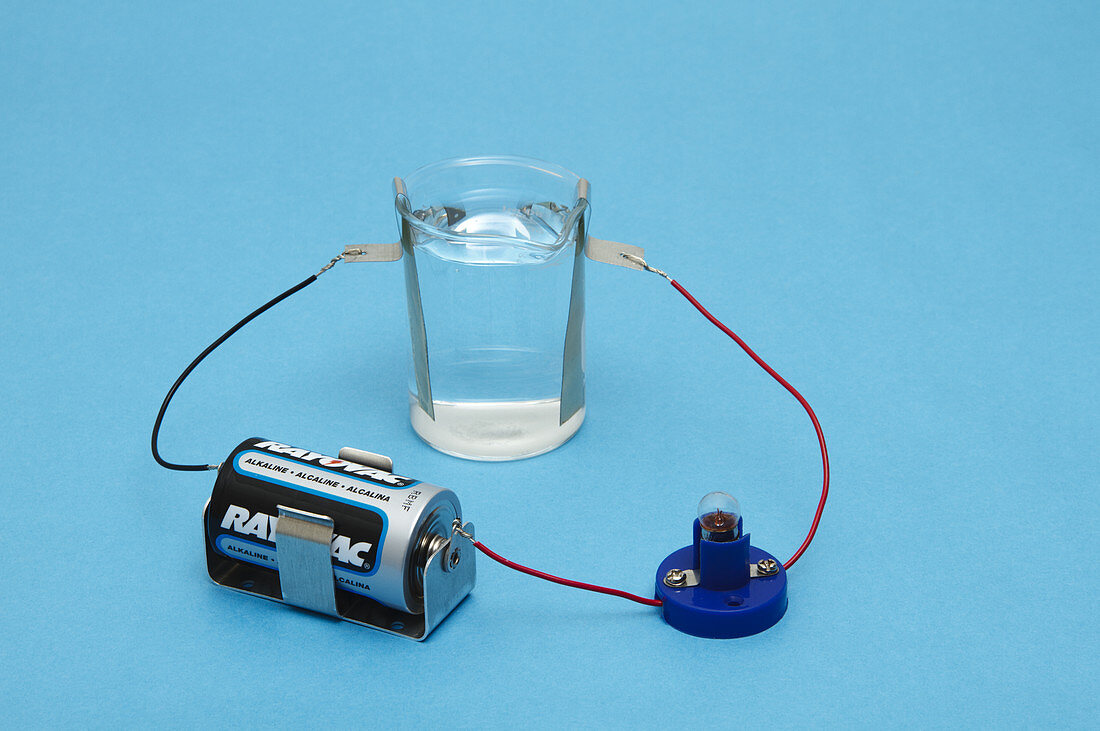
| Electrical conductivity. A circuit consisting of a battery,light bulb and two electrodes in a beaker is used to illustrate electrical conductivity. When the beaker is filled with water,the bulb does not light up because pure water is a very poor conductor (this photo 1). When table salt (sodium chloride,NaCl) is added to water,it dissociates into Na+ and Cl- ions that carry electric charges in the solution and the bulb lights up. When sugar is added to water,it dissolves but does not form ions,so the solution does not conduct electricity and the bulb does not light up. Photo 1 of 3 | |
| Lizenzart: | Lizenzpflichtig |
| Credit: | Science Photo Library / Giphotostock |
| Bildgröße: | 5149 px × 3421 px |
| Modell-Rechte: | nicht erforderlich |
| Restrictions: |
|
Preise für dieses Bild ab 15 €
Universitäten & Organisationen
(Informationsmaterial Digital, Informationsmaterial Print, Lehrmaterial Digital etc.)
ab 15 €
Redaktionell
(Bücher, Bücher: Sach- und Fachliteratur, Digitale Medien (redaktionell) etc.)
ab 30 €
Werbung
(Anzeigen, Aussenwerbung, Digitale Medien, Fernsehwerbung, Karten, Werbemittel, Zeitschriften etc.)
ab 55 €
Handelsprodukte
(bedruckte Textilie, Kalender, Postkarte, Grußkarte, Verpackung etc.)
ab 75 €
Pauschalpreise
Rechtepakete für die unbeschränkte Bildnutzung in Print oder Online
ab 495 €
